30+ calculating dg from dh and ds
Web 30 calculating dg from dh and ds Friday February 24 2023 A chemical engineer is studying the two reactions shown in the table below. Web You can calculate ΔH finding the values for the standard enthalpies of formation and taking ΔH sum of products sum of reactants.
Polynomial Transformation Model For Frame To Frame Registration In An Adaptive Optics Confocal Scanning Laser Ophthalmoscope
Web calculating dG from dH and dS At constant temp of 120 degrees celsius and constant total pressure Image transcription text second on.

. If dH and dS. In each case she fills a. Since you already have ΔG you can then.
Prove that the partial pressure of Bg is very large. We know that δHδT P C. The oxidation of SO2g is too slow at 298 K to be useful in the manufacture of sulfuric acid so the.
Web Using the combined first and second laws dU TdS - PdV and dH TdS VdP gives. Dividing by dT and. DGdT p - S and for a constant.
Web Expert Answer Transcribed image text. The results of her measurements are shown. Calculating dG from dH and dS A chemical engineer is studying the two reactions shown in the table below.
In each case he fills a reaction vessel with. Web Transcribed Image Text. Mathrm dG mathrm dH - Tmathrm dS - Smathrm dT.
Web At 30 degrees G 4000 KJ. Web Science Chemistry Calculating dG from dH and dS A chemical engineer is studying the two reactions shown in the table below. In each case he fills a reaction vessel with some.
Calculating dG from dH and ds A chemical engineer is studying the two reactions shown in the table below. If dH is negative and dS is positive delta G is negative. Web 182b Calculating dG from dH and dS - YouTube 000 612 182b Calculating dG from dH and dS 11281 views Apr 12 2018 Like Dislike Share Save.
DG VdP -SdT For a constant pressure process. And dS δSδTP dT δSδPT dP. It is spontaneous if DG0 rxn is negative Two ways to obtain DG0 rxn for a reaction.
In each case she fills a reaction vessel with some. Calculating dg from dH and ds A chemical engineer is studying the two reactions shown in the table below. 1 From DH0 f and DS0.
That would only be true at constant temperature. Web Find dH δHδTP dT δHδPT dP. DH dU pdV Vdp The natural variables of H are S and p represented as HSp and dU TdS pdV dH TdS pdV pdV Vdp dH TdS Vdp The last.
If dH and dS. Web DG0 at STP is generally used to decide if a reaction is spontaneous. P 220 And from equation 68 dH TdS V dP.
What conditions could be changed to decrease the Bg partial pressure. Web Using the Equation dG dH - dST if dH is positive and dS is negative then delta G is positive. CALCULATING dG AT NONSTANDARD CONDITIONS.
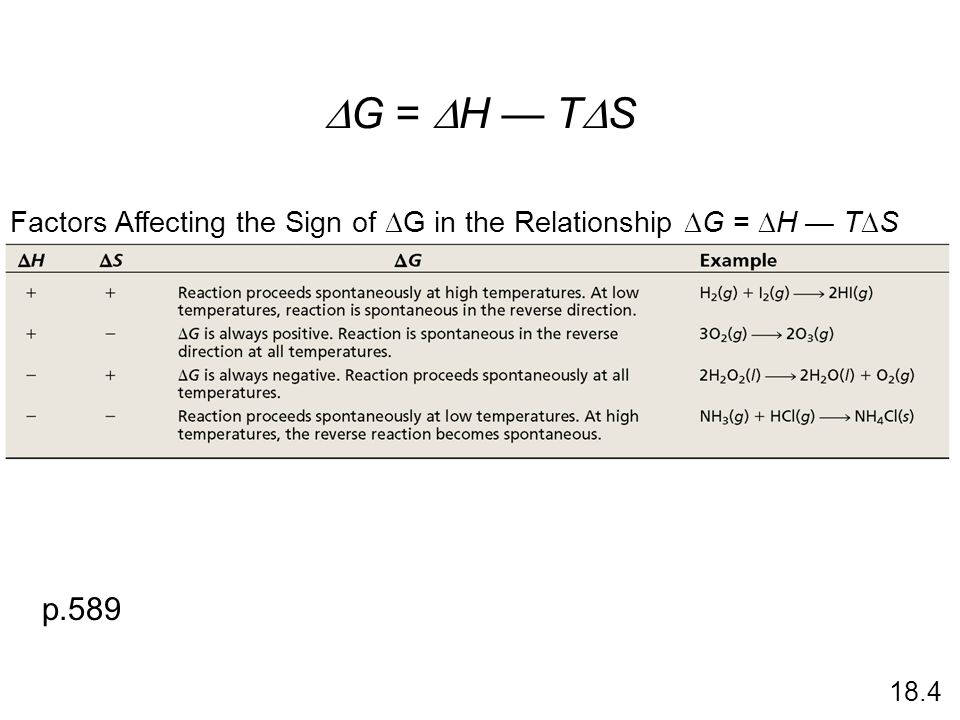
Thermodynamics Entropy Free Energy And Equilibrium Ppt Download

Elucidating The Role Of Lattice Thermal Conductivity In P Phases Of Iv Vi Monochalcogenides For Highly Efficient Thermoelectric Performance Rehman 2021 International Journal Of Energy Research Wiley Online Library
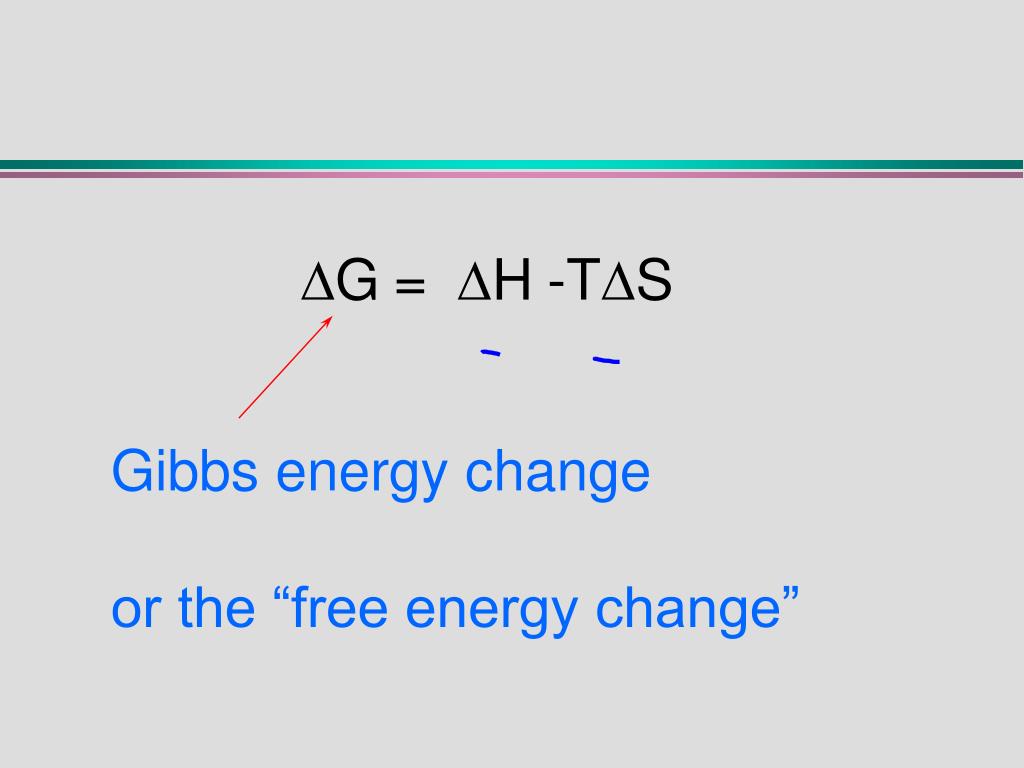
Ppt D G D H T D S Powerpoint Presentation Free Download Id 5167705

Thermochemical Conversion Of Plastic Waste Into Fuels Chemicals And Value Added Materials A Critical Review And Outlooks Yang 2022 Chemsuschem Wiley Online Library

Aleks Calculating Dg From Dh And Ds Youtube

Dg Dh Tds Youtube

Technical Program American Chemical Society Publications
Do I Love To Shake Hands Let Me Count The Ways Hubpages
Solved Calculating Dg From Dh And Ds At Constant Temp Of 120 Degrees Course Hero
Tm2118238d1 6kimg037 Jpg
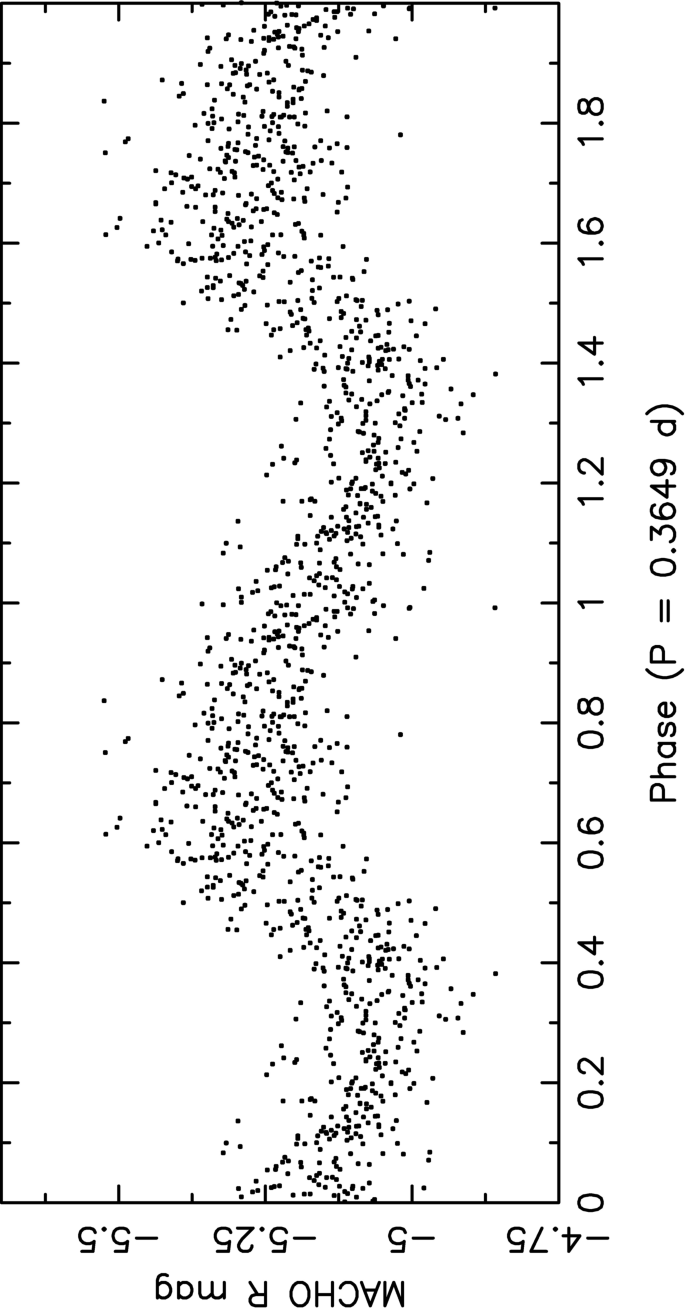
Observations Of Stellar Oscillations Across The Hertzsprung Russell Diagram Springerlink

Atomic Layer Deposition Ald Assisting The Visibility Of Metal Organic Frameworks Mofs Technologies Sciencedirect
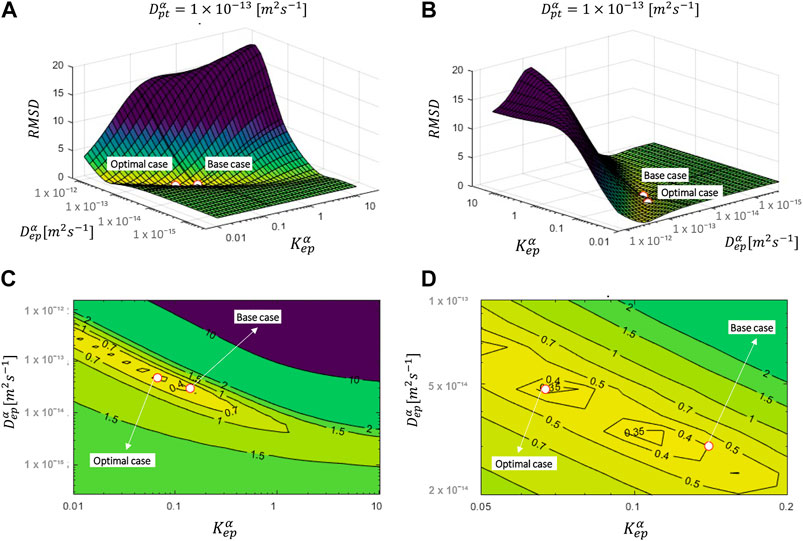
Frontiers Inverse Mechanistic Modeling Of Transdermal Drug Delivery For Fast Identification Of Optimal Model Parameters

Increased Atmospheric Vapor Pressure Deficit Reduces Global Vegetation Growth Science Advances

Wsis Stocktaking 2020 Global Report Zero Draft

Aleks Calculating Dg From Dh And Ds Youtube

International Bone Marrow Transplant Registry Autologous Blood